Educational Research Methods

A site to support teaching and learning...

Exploring conceptual integration in student thinking: evidence from a case study
Qualitative data analysis examples: These short extracts from published research papers are provided to support class discussion.
Taber, K. S. (2008). Exploring conceptual integration in student thinking: evidence from a case study. International Journal of Science Education, 30(14), 1915-1943. doi: 10.1080/09500690701589404
Abstract
Two reasons are suggested for studying the degree of conceptual integration in student thinking. The linking of new material to existing knowledge is an important aspect of meaningful learning. It is also argued that conceptual coherence is a characteristic of scientific knowledge and a criterion used in evaluating new theories. Appreciating this ‘scientific value’ should be one objective when students learn about the nature of science. These considerations imply that students should not only learn individual scientific models and principles, but should be taught to see how they are linked together. The present paper describes the use of an interview protocol designed to explore conceptual integration across two college-level subjects (chemistry and physics). The novelty here is that a single interview is used to elicit explanations of a wide range of phenomena. The potential of this approach is demonstrated through an account of one student’s scientific thinking, showing both how she applied fundamental ideas widely, and also where conceptual integration was lacking. The value and limitations of using this type of interview as one means for researching conceptual integration in students’ thinking are discussed.
Analysis of the Case
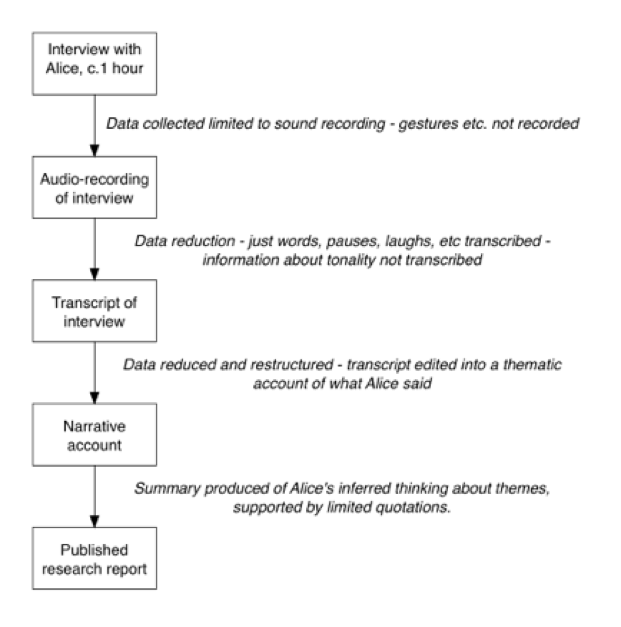
"Alice (an assumed name) was the first student to be interviewed. She had achieved the top grade in the ‘double science’ option (a ‘broad and balanced’ science course) of the school-leaving examination, and was studying biology and German, as well as chemistry and physics (see Table 1). The interview took almost exactly one hour. After the interview, Alice described the interview process as ‘challenging’. As Pope and Denicolo (1986) have discussed, the role of the analyst in qualitative work involves balancing the importance of detailed accounts directly drawing upon the words and ideas of the informants, with the need to provide reports that are concise and presented in ways to help the reader. The building of the case study took place in two stages, following a general approach used in the author’s previous research (e.g., Taber, 1995).
Firstly the interview transcript was reworked into a narrative account of the interview based around Alice’s verbatim responses, but following the chronology of the interview schedule in the order of the questions. This stage of analysis is largely descriptive—similar to the initial ‘open coding’ stage used in much qualitative analysis (Glaser & Holton, 2004). The main purpose was to convert the format from a transcript into a narrative form to aid readability."
pp.1925-1926
"The total length of the narrative account was a little over 2,300 words. The next stage of the analysis involved reorganising the case material into themes in terms of the main concepts used in Alice’s explanations (see the next section). This process produced a case account that was reduced (in this case to about 1,000 words), and which summarises the ways Alice used ideas in her interview. The case account is presented in the next section to illustrate the range of information that was elicited in a single interview tightly structured by the schedule presented in the Appendix. p.1926
Case Study of Conceptual Understanding across Physics and Chemistry Topics
…
Interactions between Charges
According to Alice, a balloon could be attached to a wall by charging, so that there was a ‘glue effect’ with an attraction between areas of positive and negative charge. A negatively charged balloon could be attached to a neutral wall because it was relatively positive, although an individual nuclear proton would not be attracted to a neutron in this way. Static electricity could make someone’s hair stand on end as the hairs would have the same charge and be forced away from each other.
Alice recognised attractions between charges at atomic level; for example, suggesting that a positive hydrogen ion would attract a negative fluorine species. She described van der Waals’ forces in terms of momentary dipoles due to the shifting electron clouds around molecules: again attraction between positives and negatives. Delocalised electrons in a metal acted as a glue between the positive nuclei. In ice there was a lattice of dipoles that have lined up, positives to negatives. These dipoles could attract and pull away parts of the sodium chloride so that it would dissolve. An atom is also held together by the attractions between positive and negative charges. Electrons could be attracted away from atoms. The difficulty increased with each electron removed as the effective nuclear charge increased the amount of charge acting on each remaining electron—although the shells of electrons could lead to shielding making the situation more complicated.
p.1927
…
Discussion of the Case
…
"Finally, it is worth considering her explanation for why a balloon rubbed on a jumper is able to remain attached to a wall. This is a ‘party trick’ familiar to most children, but probably not specifically discussed in many science classrooms. Alice recognised this as being an electrostatic effect:
some sort of interaction if you like with the electrons and things, and you have a positive and negative charge which allows, a glue effect if you like, attraction between two areas, one of positive and one of negative.
The charging was easily explained as ‘when you’re rubbing the balloon you’re transferring electrons either onto it or away from it’. To explain why the balloon would stick to the wall, Alice proposed that ‘because you’ve got opposite charges, you’ve got the say negatively charged balloon, and then your positively charged wall’. Although the wall ‘hasn’t had anything done to it as such’, Alice suggested that ‘maybe in comparison to your very negatively charged balloon, it’s still likely to attract.’ Alice agreed that she was suggesting that ‘it’s relative’; that because the neutral object is positive by comparison with the negative object, they are effectively both charged."
p.1930
…
"Alice saw NaCl as a molecular structure, and explained the integrity of the solid in terms of ‘strong enough … intermolecular forces holding things together’. She suggested that these forces might be ‘van der Waals’ forces’ which were where:
you’ve got if you like an electron cloud between, surrounding … each molecule, and as these clouds don’t stay in one fixed place, there’s always going to be erm sort of momentary areas of dipole. And that’s where you get your positive and negatives attracting each other again.
So in the context of intermolecular bonding, Alice discussed how neutral species could be attracted due to induced dipoles. However, she did not consider a possibility along these lines to explain how the charged balloon could somehow have an attraction with a neutral wall. Here the potential linkage was missed.
pp.1930-1931
(Brief extracts such as these can only give you a flavour of a study. You can use the citations to access the full papers to explore the extracts here in the contexts of the full studies.)
This is a personal site of Keith S. Taber to support teaching of educational research methods.
(Dr Keith Taber is Professor of Science Education at the University of Cambridge.)
2016
Taber, K. S. (2013). Classroom-based Research and Evidence-based Practice: an introduction (2nd ed.). London: Sage.
Figure from Taber, K. S. (2013).
